Explain Difference Between Mass Number and Atomic Number
Significance of Avogadros Number. Due to massenergy equivalence this corresponds to a rest energy of 0511 MeV.

Atomic Number Atomic Mass Number Science Mass Number Atomic Number Science
GCSE Combined Science Physics Combined Science learning resources for adults children parents and teachers.

. Give the difference between isobars and isotopes. Atomic number 50 mass number 118. FlexBook Platform FlexBook FlexLet and FlexCard are registered trademarks of CK-12 Foundation.
For example isobars boron-12 oxygen-12 nitrogen-12 and carbon-12 exist. The number of protons in the nucleus of a tin atom is 50 while the number of neutrons in the nucleus is 68. The unit of measurement is grams per mole.
The difference between isobars and isotopes is given below. The Atomic mass unit is defined as the 112 th weight of the mass of one carbon atom. What are the atomic number and the mass number of this isotope.
The nucleons and atomic mass of the isobars are the same but the number of protons and neutrons is different. For example the atomic mass unit of Hydrogen is 100794 amu. The ratio between the mass of a proton and that of an electron is about 1836.
Unbinilium also known as eka-radium or simply element 120 is the hypothetical chemical element in the periodic table with symbol Ubn and atomic number 120. Unbinilium and Ubn are the temporary systematic IUPAC name and symbol which are used until the element is discovered confirmed and a permanent name is decided uponIn the periodic table of the. The invariant mass of an electron is approximately 9109 10 31 kilograms or 5489 10 4 atomic mass units.
Because the mass number is 235 then the number of neutrons in the nucleus is 235 92 or 143. The notion of molar mass can be used to calculate how many grams of substance are necessary when we know the number of moles required. The molar mass also known as molecular weight is the sum of all the atoms that make up a mole of a molecules total mass in grams.
Now to calculate the ability of a single particle atom electron molecule to say carry. Atomic mass of H 1u. Isobars have a comparable Atomic mass.
In the atomic level substances are measured as per atomic mass unit.
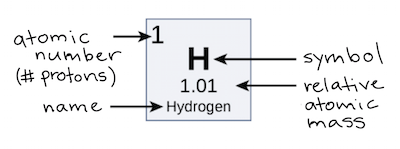
Atomic Number Atomic Mass And Isotopes Article Khan Academy

What Is An Atomic Number Definition And Examples
Difference Between Atomic Number And Mass Number Definition Explanation With Examples
No comments for "Explain Difference Between Mass Number and Atomic Number"
Post a Comment